Pioneros 30 años de trasplantes de células madre hematopoyéticas en Puebla, México
Rev Hematol Mex. 2023; 24 (2): 37-45. https://doi.org/10.24245/rev_hematol.v24i2.9223
Javier Garces Eisele,1,2 Juan Carlos Olivares Gazca2,3,4
1 Laboratorios Clínicos de Puebla, Puebla, México.
2 Universidad Popular Autónoma del Estado de Puebla, Puebla, México.
3 Centro de Hematología y Medicina Interna de Puebla, Puebla, México.
4 Universidad de las Américas Puebla, Puebla, México.
“If I have seen further, it is by standing on the shoulders of giants”
Isaac Newton, 1676
The history of hematopoietic stem cell transplantation is marked by remarkable milestones. In the mid-20th century, Dr. E. Donnall Thomas achieved a landmark breakthrough by successfully performing the first bone marrow transplant between identical twins in 1956 (Thomas et al., 1957). This pioneering work laid the foundation for the exploration of hematopoietic stem cells and their role in treating diseases of the blood and immune system. Dr. Thomas’ achievements earned him the Nobel Prize in Physiology or Medicine in 1990 – a testament to his groundbreaking contributions. Dr. George Mathé explored the transplantation of bone marrow cells from healthy donors to treat patients accidentally irradiated at high dose, expanding the possibilities of this life-saving technique (Mathé et al., 1959).
At the time they performed the first transplants surprisingly little was known about hematopoietic stem cells, immune responses to transplants or the complex human leucocyte antigen system. The work of Jean Dausset, whose discovery of the human leukocyte antigen (HLA) system revolutionized our understanding of tissue compatibility for transplantation (Dausset J, 1958). This breakthrough, for which Dausset was awarded the Nobel Prize in Physiology or Medicine in 1980, opened the door to safer and more successful organ and stem cell transplantation. In April 1960, Dr. Álvaro Gómez-Leal, presented during the first meeting of the Agrupación Mexicana para el Estudio de la Hematología, A.C., data on a transplant of allogeneic stem cells in a patient with acute leukemia done in Monterrey, Mexico: the patient received high-dose chemotherapy followed by stem cells from the bone marrow of his brother, improving and obtaining remission for months but relapsing and subsequently died. This was the first report of a hematopoietic stem cell transplantation (HSCT) conducted in Mexico, only 4 years after the pioneer work by E. Donnall Thomas in Cooperstown, New York, USA (Ruiz-Argüelles et al., 2021). Twenty years later, in 1980, Ricardo Sosa and his coworkers at the Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán conducted and published another HSCT (Sosa-Sánchez et al., 1980). As with many countries embarking on this procedure, there were challenges related to transplant-related morbidity and mortality. It was only until 1988 when the same group could report on their first successful bone marrow transplant (León-Rodríguez et al., 1992). In the late 1980s and early 1990s, the field of HSCT was still evolving globally. Changes in the sources and handling of hematopoietic stem cells were introduced. Stem cells were susceptible to cryo-preservation (Stiff et al., 1987). Umbilical cord blood was recognized as an alternative source of hematopoietic stem cells (Gluckman et al., 1989). Hematopoietic stem cells could be harvested easier from peripheral blood after mobilization with G-CSF (Sheridan et al., 1992). Since then, hematopoietic stem cells, with their unique ability to differentiate into various blood cell types, have proven to be a revolutionary tool in the treatment of numerous hematological disorders. The successful application of HSCT for diseases such as leukemia, lymphoma, myeloma, and inherited blood disorders underscored its broad applicability in clinical practice (Snowden et al., 2022).
The efficacy of HSCT extends to conditions beyond these well-established diseases. Research explored its use in autoimmune disorders with excellent results. HSCT offers a way to reset the immune system, providing a novel approach to treating conditions such as multiple sclerosis, systemic sclerosis, and rheumatoid arthritis (Swart et al., 2017; Alexander et al., 2021).
Furthermore, this approach is being investigated for its potential in non-hematological diseases. Promising preclinical studies suggest that hematopoietic stem cells could be harnessed to target genetic disorders, metabolic diseases, and neurodegenerative conditions. By utilizing the stem cells’ remarkable ability to differentiate into various cell types, researchers are envisioning a future where hematopoietic stem cell transplantation could address a broader spectrum of medical challenges (Chen et al., 2021).
However, back in the early 1990s, while high-income countries were making strides in research and application, access to this life-saving treatment in middle-income regions like Mexico was limited mainly due to economic constraints and a lack of specialized facilities (Gale et al., 2016). These centers were concentrated in Mexico City. The development of infrastructure and expertise for HSCT was a gradual process. The cost of HSCT was high. Given the limited economic resources available to many in Mexico, not everyone could afford the procedure. Recognizing early this disparity since he initiated the program in 1993 (Ruiz-Argüelles et al., 1993), the group of Ruiz-Argüelles has made it his mission to increase accessibility of this pioneering therapy by focusing on the cost-effectiveness of transplant procedures without compromising on quality and outcomes. Figure 1
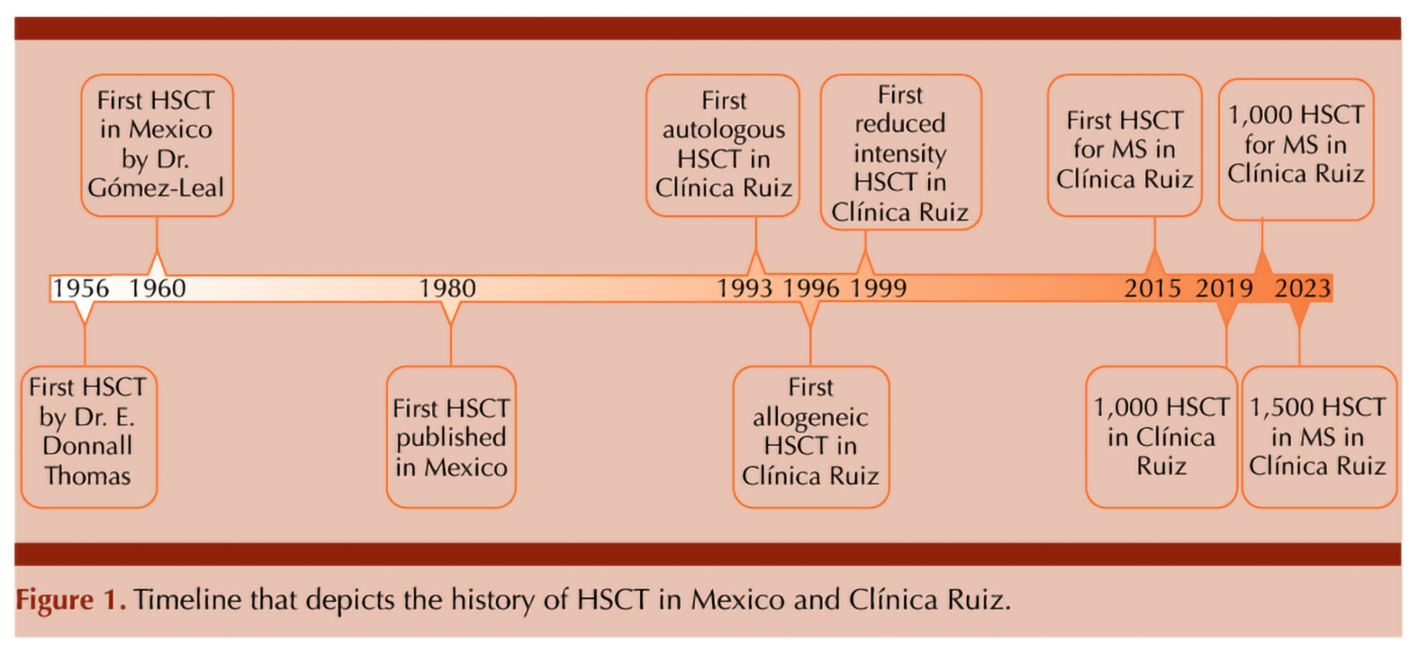
This goal was approached by the following measures:
1. Outpatient transplantation: Challenging conventional norms, one of the most significant changes introduced by Ruiz-Argüelles and coworkers was the concept of outpatient HSCT. Instead of keeping patients hospitalized for extended periods (which is standard in many high-income countries), he developed protocols where patients could receive transplants on an outpatient basis. This reduced significantly the costs associated with prolonged hospital stays while maintaining patient safety and outcomes are even better reducing the incidence of graft versus host disease (GVHD) and infections rate decreases. (Ruiz-Argüelles et al., 1998; Gómez-Almaguer et al., 2000; Ruiz-Argüelles et al., 2002).
2. Modified conditioning regimens: Increasing safety while maintaining efficacy of HSCT was the goal of modifying the conditioning regimens; nowadays they have been classified according to the duration of cytopenia and the requirement for stem cell support to three categories: (1) myeloablative conditioning, (2) reduced-intensity conditioning, and (3) non-myeloablative conditioning. As myeloablative regimens cause irreversible cytopenia, failure in engraftment is lethal. On the other hand, non-myeloablative regimens and reduced-intensity conditioning regimens may recover as cytopenia may not be irreversible (Bacigalupo et al., 2009). The first one relies on tumor destruction partly by chemotherapy as well as by the GVHD effect, while the second relies exclusively on GVHD. Ruiz-Argüelles, Gómez-Almaguer and coworkers refined the conditioning regimens used and achieved a delicate balance between safety (reduced degree and duration of cytopenia and thus the risk of complications) and efficacy, optimizing engraftment rates while minimizing adverse effects and, importantly, the risk of a graft failure (Gómez-Almaguer et al., 2000). These reduced conditioning regimens eliminated the need of laminar flow rooms and HEPA filters and thus were the cornerstone for the ability to perform the transplants on an outpatient basis (Ruiz-Argüelles et al., 2022).
3. Utilization of biosimilar drugs: When available and of good quality, the use of biosimilar drugs can reduce further the costs of transplantation. The groups of Ruiz-Argüelles and Gómez-Almaguer have been advocates for the appropriate use of national biosimilars in the HSCT process, ensuring of course, that cost savings do not compromise the efficacy of the treatment (León-González et al., 2017; Ruiz-Argüelles et al., 2022; Gómez-Almaguer et al., 2022; Gallardo-Pérez et al., 2023).
4. Streamlined procedures: By refining and streamlining the transplantation procedures, Ruiz-Argüelles et al. ensured that resources were used optimally, avoiding wastages and unnecessary expenses. One of these avoidable expenses is the cryopreservation of mobilized hematopoietic stem cells in autologous HSCTs (Ruiz-Argüelles et al., 1995; Gómez-Almaguer et al., 1997; Ruiz-Argüelles et al., 1998). Engraftment rate was even higher and thus reduced further the associated risks, in more than 70% of cases the procedure could be completed totally on an outpatient basis (Ruiz-Argüelles et al., 2008).
5. Haploidentical transplants: Recognizing the challenges in finding suitable unrelated donors and costs in obtaining a suitable graft, the groups of Gómez-Almaguer and Ruiz-Argüelles explored the potential of haploidentical transplants, where the donor is a half-match to the recipient. The advantages were evident: a donor is almost always (in 95% of the cases or higher) available immediately, it can be better selected based on natural killer cell alloreactivity, and the donor is available for repeated infusions, among others. By adapting chemotherapy to mitigate graft rejection and GVHD, he expanded transplant options for patients without fully matched donors (Ruiz-Argüelles et al., 2015; González-Llano et al., 2016). Again, the procedure could be safely conducted on an outpatient basis (Murrieta-Álvarez et al., 2021; Murrieta-Álvarez et al., 2021b). Very recently, the groups of Ruiz-Argüelles and Gómez-Almaguer have shown that the doses of post-transplant cyclophosphamide employed in the conduction of haploidentical transplantation, can be safely reduced to 50% of the original proposed doses (Olivares-Gazca et al., 2023)
6. World class quality HSCT in Mexico: The international community of bone marrow experts have analyzed the outcomes obtained by Ruiz-Argüelles and his team using his modified protocol to conduct HSCT. Nowadays, other programs in the world are replicating the method. On the other hand, this recognition by his pairs gave him the opportunity to share his experience in multiple meetings in different countries and very recently, the Ruiz-Argüelles program has been certified with the first step of two of the FACT-JACIE accreditation, being the third program in Latin-America to achieve this quality certification and the first one as a fully outpatient program.
There is an ever-increasing number of alternatives to HSCT for treating onco-hematological diseases, whose spiraling costs (Green et al., 2016; Weisdorf et al., 2017), however, are prohibitive to most patients in middle-income countries. Even conventional HSCT are out of reach to many patients in Mexico. The non-myeloablative allo-HSCT procedure cuts the cost (Ruiz-Argüelles, 2010) and has stimulated the instantiation of similar programs and influenced the development of simplified HSCT programs in other middle-income countries (Schroeder et al., 2011; Ramzi et al., 2012; Bittencourt et al., 2019; Aljurf et al., 2020; Bekadja et al., 2021; Ahmed Al-Anazi et al., 2023). Built on his ample experience, Ruiz-Argüelles and coworkers have elaborated guidance on how to start and stepwise develop an affordable HSCT program in resource-limited settings (Ruiz-Argüelles GJ, 2020; Ruiz-Argüelles et al., 2021; Ruiz-Argüelles et al., 2022).
Drawing from his experience in HSCT, the groups of Ruiz-Argüelles and Gómez-Almaguer began exploring further the potential of an autologous HSCT (aHSCT) for patients with autoimmune conditions such as multiple sclerosis (MS). His vision was to offer an alternative treatment option for patients who were not adequately responding to conventional therapies or experiencing significant disease progression (Ruiz-Argüelles et al., 2017; Ruiz-Argüelles & Gómez-Almaguer, 2017).
MS is a chronic autoimmune disease that affects the central nervous system, causing inflammation, demyelination, and neuronal damage. This leads to a wide range of neurological symptoms, including asthenia, balance disorder, fatigue, and cognitive impairment. MS is a complex and unpredictable disease, and while there are disease-modifying therapies available, not all patients respond adequately to these treatments (Dobson & Giovannoni, 2019; Piehl F, 2021; Kuhlmann et al., 2023).
Treatment by aHSCT aims at “resetting” the immune system by controlling autoreactive clones and instilling immunological tolerance upon the reestablishment of the immune system. The results showed encouraging evidence of disease stabilization and even improvement. Patients who underwent aHSCT reported a reduction in relapse rates, disability progression, and inflammatory disease activity, leading to an improved overall quality of life (Ruiz-Argüelles et al., 2018; Ruiz-Argüelles et al., 2019; Murrieta-Álvarez et al., 2021c; Olivares-Gazca et al., 2022; Olivares-Gazca et al., 2022b; Sánchez-Bonilla et al., 2023).
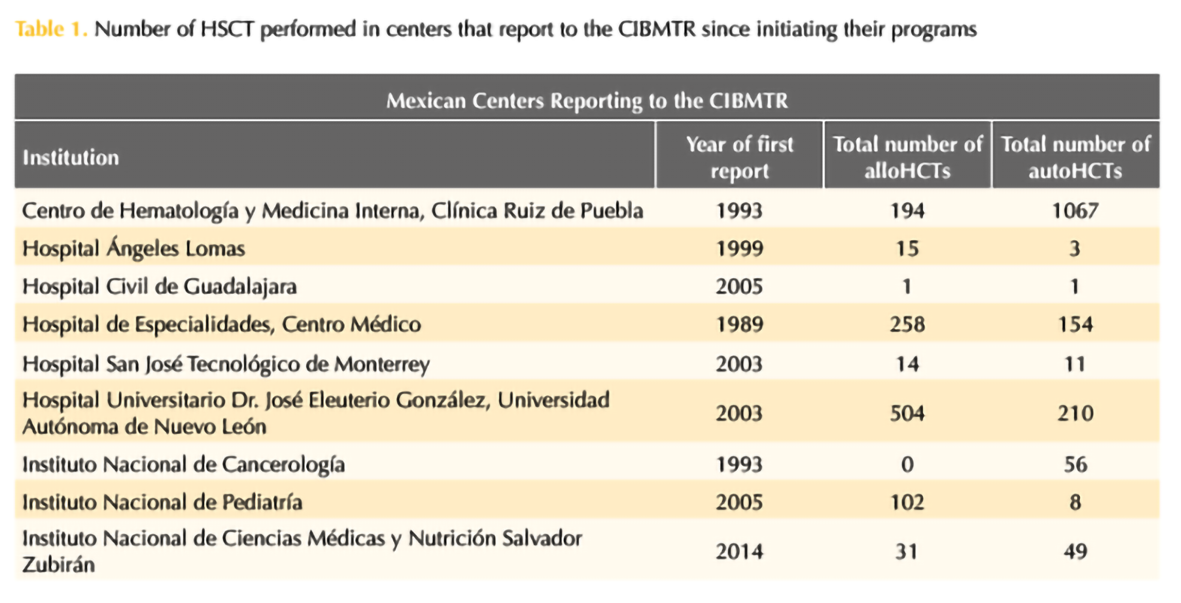
Four years ago, the 1000th HSCT was performed by the group of Ruiz-Argüelles (Gómez-Cruz et al., 2019; Maziarz RT, 2020) and 2 years ago it has been the 1000th aHSCT in patients with MS and other autoimmune disorders, highlighting the success of this program (Murrieta-Álvarez et al., 2021). In this year, the program led by Ruiz-Argüelles accomplished the first 1,500 patients engrafted with MS as depicted in Table 1. The work by both Gómez-Almaguer and Ruiz-Argüelles has been acknowledged by the Center for International Blood and Marrow Transplantation Research (CIBMTR), who presented them with the Distinguished Service Award in 2017, as a reflection of a lifetime commitment with improving the accessibility of patients to high-cost therapies in an underdeveloped country always thinking “outside the box”. Now, México as a country and Monterrey and Puebla as cities are on the map as the most important centers for HSCT in the country and a reference to the world.
REFERENCES
1. Thomas ED, Lochte HL, Lu WC, Ferrebee JW. Intravenous infusion of bone marrow in patients receiving radiation and chemotherapy. N Engl J Med 1957; 257: 491-6. doi: 10.1056/NEJM195709122571102.
2. Mathé G, Jammet H, Pendic B, Schwarzenberg L, Duplan JF, Maupin B, Latarjet R, Larrieu MJ, Kalic D, Djukic Z. Transfusions et greffes de moelle osseuse homologue chez des humains irradies a hautes dose accidentellement. Rev Fr Etudes Clin Biol 1959; 4 (3): 226-38.
3. Dausset J. Iso-leuco-anticorps. Acta Haematol 1958; 20 (1-4): 156-66. doi: 10.1159/000205478.
4. Sosa-Sánchez R, Córdova MS, Labardini JR, Chávez-Peón F. Trasplante de médula ósea en anemia aplástica. Reporte del primer caso en México. Rev Invest Clin Mex 1980; 32: 49-55.
5. León-Rodríguez E, Sosa-Sánchez R. Trasplante de médula ósea en México. Informe del primer caso exitoso en leucemia aguda mieloblástica. Grupo de Trasplante Medular Óseo del INNSZ. Rev Invest Clin 1992; 44 (3): 383-6.
6. Stiff PJ, Koester AR, Weidner MK, Dvorak K, Fisher RI. Autologous bone marrow transplantation using unfractionated cells cryopreserved in dimethylsulfoxide and hydroxyethyl starch without controlled-rate freezing. Blood 1987; 70 (4): 974-8.
7. Gluckman E, Broxmeyer HA, Auerbach AD, Friedman HS, Douglas GW, Devergie A, Esperou H, Thierry D, Socie G, Lehn P, et al. Hematopoietic reconstitution in a patient with Fanconi’s anemia by means of umbilical-cord blood from an HLA-identical sibling. N Engl J Med 1989; 321 (17): 1174-8. doi: 10.1056/NEJM198910263211707.
8. Sheridan WP, Begley CG, Juttner CA, Szer J, To LB, Maher D, McGrath KM, Morstyn G, Fox RM. Effect of peripheral-blood progenitor cells mobilised by filgrastim (G-CSF) on platelet recovery after high-dose chemotherapy. Lancet 1992; 339 (8794): 640-4. doi: 10.1016/0140-6736(92)90795-5.
9. Snowden JA, Sánchez-Ortega I, Corbacioglu S, Basak GW, Chabannon C, de la Camara R, Dolstra H, Duarte RF, Glass B, Greco R, Lankester AC, Mohty M, Neven B, de Latour RP, Pedrazzoli P, Peric Z, Yakoub-Agha I, Sureda A, Kröger N; European Society for Blood and Marrow Transplantation (EBMT). Indications for haematopoietic cell transplantation for haematological diseases, solid tumours and immune disorders: current practice in Europe, 2022. Bone Marrow Transplant 2022; 57 (8): 1217-1239. https://doi.org/10.1038/s41409-022-01691-w.
10. Swart JF, Delemarre EM, van Wijk F, Boelens JJ, Kuball J, van Laar JM, Wulffraat NM. Haematopoietic stem cell transplantation for autoimmune diseases. Nat Rev Rheumatol 2017; 13 (4): 244-256. https://doi.org/10.1038/nrrheum.2017.7.
11. Alexander T, Greco R, Snowden JA. Hematopoietic stem cell transplantation for autoimmune disease. Annu Rev Med 2021; 72: 215-228. doi: 10.1146/annurev-med-070119-115617.
12. Chen J, Luo L, Tian R, Yu C. A review and update for registered clinical studies of stem cells for non-tumorous and non-hematological diseases. Regen Ther 2021; 18: 355-362. doi: 10.1016/j.reth.2021.09.001.
13. Gale RP, Seber A, Bonfim C, Pasquini M. Haematopoietic cell transplants in Latin America. Bone Marrow Transplant 2016; 51 (7): 898-905. doi: 10.1038/bmt.2016.35.
14. Ruiz-Argüelles GJ, Ruiz-Argüelles A, Alemán-Hoey DD, Arizpe Bravo D, Martin-López A, Ocejo Rodríguez A. Autotransplante en leucemia aguda de células totipotenciales movilizadas con filgrastim [Autotransplantation in acute leukemia using totipotent cells mobilized with filgrastim]. Rev Invest Clin 1993; 45 (5): 479-80.
15. Ruiz-Argüelles GJ, Ruiz-Argüelles A, Pérez-Romano B, Marín-López A, Delgado-Lamas JL. Non-cryopreserved peripheral blood stem cells autotransplants for hematological malignancies can be performed entirely on an outpatient basis. Am J Hematol 1998; 58 (3): 161-4. doi: 10.1002/(sici)1096-8652(199807)58:3<161::aid-ajh1>3.0.co;2-p.
16. Gómez-Almaguer D, Ruiz-Argüelles GJ, Ruiz-Argüelles A, González-Llano O, Cantú OE, Hernández NE. Hematopoietic stem cell allografts using a non-myeloablative conditioning regimen can be safely performed on an outpatient basis: report of four cases. Bone Marrow Transplant 2000; 25 (2): 131-3. doi: 10.1038/sj.bmt.1702100.
17. Ruiz-Argüelles GJ. Allogeneic stem cell transplantation using non-myeloablative conditioning regimens: results of the Mexican approach. Int J Hematol 2002; 76 Suppl 1: 376-9. doi: 10.1007/BF03165287.
18. Bacigalupo A, Ballen K, Rizzo D, Giralt S, Lazarus H, Ho V, Apperley J, Slavin S, Pasquini M, Sandmaier BM, Barrett J, Blaise D, Lowski R, Horowitz M. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant 2009; 15 (12): 1628-33. doi: 10.1016/j.bbmt.2009.07.004.
19. Ruiz-Argüelles GJ, Seber A, Ruiz-Delgado GJ. Conducting hematopoietic stem cell transplantation in low and middle income countries. Hematology 2022; 27 (1): 809-812. doi: 10.1080/16078454.2022.2105513.
20. León-González M, León-Peña AA, Vallejo-Villalobos MF, Nuñez-Cortés AK, Ruiz-Argüelles A, Ruiz-Argüelles GJ. Mexican biosimilar filgrastim for autologous hematopoietic stem cell mobilization and transplantation. Rev Invest Clín 2017; 68 (4): 181-3. doi: 10.1080/16078454.2022.2105513.
21. Gómez-Almaguer D, Gómez-De León A, Colunga-Pedraza PR, Cantú-Rodríguez OG, Gutierrez-Aguirre CH, Ruíz-Arguelles G. Outpatient allogeneic hematopoietic stem-cell transplantation: a review. Ther Adv Hematol 2022; 13: 20406207221080739. doi: 10.1177/20406207221080739.
22. Gallardo-Pérez MM, Gale RP, Reyes-Cisneros OA, Sánchez-Bonilla D, Fernández-Gutiérrez JA, Stock W, Murrieta-Álvarez I, Olivares-Gazca JC, Ruiz-Delgado GJ, Fonseca R, Ruiz-Argüelles GJ. Therapy of childhood acute lymphoblastic leukemia in resource-poor geospaces. Front Oncol 2023; 13: 1187268. doi: 10.3389/fonc.2023.1187268.
23. Ruiz-Argüelles GJ, Ruiz-Argüelles A, Pérez-Romano B, Marín-López A, Larregina-Díez A, Apreza-Molina MG. Filgrastim-mobilized peripheral-blood stem cells can be stored at 4 degrees and used in autografts to rescue high-dose chemotherapy. Am J Hematol 1995; 48 (2): 100-3. https://doi.org/10.1002/ajh.2830480206.
24. Gómez-Almaguer D, Ruiz-Argüelles GJ, Piñeiro LA, Ruiz-Argüelles A. Dos casos de trasplante heterólogo con sangre periférica [Two cases of heterologous transplantation with peripheral blood]. Rev Invest Clin 1997; 49 (1): 41-5.
25. Ruiz-Argüelles GJ, Gómez-Almaguer D. Making allogeneic bone marrow transplantation available to patients in developing countries: The Mexican Experience. Open Hematol J 2008; 2 (1): 67-73. http://dx.doi.org/10.2174/1874276900802010067.
26. Ruiz-Argüelles GJ, Ruiz-Delgado GJ, González-Llano O, Gómez-Almaguer D. Haploidentical bone marrow transplantation in 2015 and beyond. Curr Oncol Rep 2015; 17 (12): 57. doi: 10.1007/s11912-015-0482-9.
27. González-Llano O, González-López EE, Ramírez-Cázares AC, Marcos-Ramírez ER, Ruiz-Argüelles GJ, Gómez-Almaguer D. Haploidentical peripheral blood stem cell transplantation with posttransplant cyclophosphamide in children and adolescents with hematological malignancies. Pediatr Blood Cancer 2016; 63 (11): 2033-7. doi: 10.1002/pbc.26131.
28. Murrieta-Álvarez I, Olivares-Gazca JC, Cantero-Fortiz Y, León-Peña AA, Priesca-Marin JM, Ruiz-Delgado GJ, Ruiz-Argüelles GJ. Haploidentical stem cell transplantation can be fully conducted on an outpatient basis. Blood 2021; 138: 4912. https://doi.org/10.1182/blood-2021-149961.
29. Murrieta-Álvarez I, Ruiz-Argüelles GJ. Bien plus Encore: Haplos indeed can be completed on an outpatient basis. Transplant Cell Ther 2021b; 27 (6): 519-520. doi: 10.1016/j.jtct.2021.03.009.
30. Green T, Bron D, Chomienne C, de Wit TD, de Haas F, Engert A, Hagenbeek A, Jäger U, MacIntyre E, Muckenthaler MU, Smand C, Sonneveld P. Costs of haematological disease high and rising. Lancet Haematol 2016; 3 (8): e353-4. doi: 10.1016/S2352-3026(16)30074-6.
31. Weisdorf D, Ruiz-Arguelles GJ, Srivastava A, Gómez-Almaguer D, Szer J. Economic challenges in hematopoietic cell transplantation: How will new and established programs face the growing costs? Biol Blood Marrow Transplant 2017; 23 (11): 1815-1816. https://doi.org/10.1016/j.bbmt.2017.07.026.
32. Ruiz-Argüelles GJ. Whither the bone marrow transplant? Hematology 2010; 15 (1): 1-3. doi: 10.1179/102453310X12583347009892.
33. Schroeder T, Fenk R, Saure C, Czibere A, Bruns I, Zohren F, Haas R, Kobbe G. The Mexican way: a feasible approach to avoid DMSO toxicity. Bone Marrow Transplant 2011; 46 (3): 469-71. https://doi.org/10.1038/bmt.2010.140.
34. Ramzi M, Zakerinia M, Nourani H, Dehghani M, Vojdani R, Haghighinejad H. Non‐cryopreserved hematopoietic stem cell transplantation in multiple myeloma, a single center experience. Clin Transplant 2012; 26 (1): 117-22. doi: 10.1111/j.1399-0012.2011.01432.x.
35. Bittencourt MCB, Mariano L, Moreira F, Schmidt-Filho J, Mendrone-Jr A, Rocha V. Cryopreserved versus non-cryopreserved peripheral blood stem cells for autologous transplantation after high-dose Melphalan in multiple myeloma: comparative analysis. Bone Marrow Transplant 2019; 54 (1): 138-141. doi: 10.1038/s41409-018-0250-1.
36. Aljurf M, Weisdorf D, Hashmi SK, Nassar A, Gluckman E, Mohty M, Rizzo D, Pasquini M, Hamadani M, Saber W, Hari P, Kharfan-Dabaja M, Majhail N, Gerges U, Hamidieh AA, Hussain F, Elhaddad A, Mahmoud HK, Tbakhi A, Othman TB, Hamladji RM, Bekadja MA, Ahmed P, Bazarbachi A, Adil S, Alkindi S, Ladeb S, Dennison D, Patel M, Lu P, Quessar AE, Okamoto S, Atsuta Y, Alhejazi A, Ayas M, Ahmed SO, Novitzky N, Srivastava A, Seber A, Elsolh H, Ghavamzadeh A, Confer D, Kodera Y, Greinix H, Szer J, Horowitz M, Niederwieser D. Worldwide Network for Blood and Marrow Transplantation (WBMT) recommendations for establishing a hematopoietic stem cell transplantation program in countries with limited resources (Part II): Clinical, technical and socio-economic considerations. Hematol Oncol Stem Cell Ther 2020; 13 (1): 7-16. doi: 10.1016/j.hemonc.2019.08.002.
37. Bekadja MA, Boumendil A, Blaise D, Chevallier P, Peggs KS, Salles G, Giebel S, Marks R, Arcese W, Milpied N, Finel H, Gorin NC. Non-cryopreserved hematopoietic stem cells in autograft patients with lymphoma: a matched-pair analysis comparing a single center experience with the use of cryopreserved stem cells reported to the European Society for Blood and Marrow Transplantation registry. Cytotherapy 2021; 23 (6): 483-487. doi: 10.1016/j.jcyt.2020.12.016.
38. Ahmed Al-Anazi K, Alshaibani Z, Kalogianidis P. An update on hematopoietic stem cell transplantation in patients with multiple myeloma [Internet]. Recent updates on multiple myeloma. IntechOpen 2023. http://dx.doi.org/10.5772/intechopen.109059.
39. Ruiz-Argüelles GJ. Lessons learned starting a bone marrow transplantation programme in a resource-constrained setting. Lancet Haematol 2020; 7 (7): e509-e510. doi: 10.1016/S2352-3026(20)30184-8.
40. Ruiz-Argüelles GJ, Gómez-Almaguer D. Lessons learned treating patients with multiple myeloma in resource-constrained settings. Curr Hematol Malig Rep 2021; 16 (1): 40-44. doi: 10.1007/s11899-021-00616-6.
41. Ruiz-Argüelles GJ, León-Peña AA, León-González M, Nuñez-Cortes AK, Olivares-Gazca JC, Murrieta-Alvarez I, Vargas-Espinosa J, Medina-Ceballos E, Cantero-Fortiz Y, Ruiz-Argüelles A, Ruiz-Delgado MA, Ruiz-Delgado RJ, Ruiz-Reyes G, Priesca-Marín M, Torres-Priego MS, Blumenkron-Marroquin D, Ruiz-Delgado GJ. A feasibility study of the full outpatient conduction of hematopoietic transplants in persons with multiple sclerosis employing autologous non-cryopreserved peripheral blood stem cells. Acta Haematol 2017; 137 (4): 214-219. doi: 10.1159/000469655.
42. Ruiz-Argüelles GJ, Gómez-Almaguer D. Hematopoietic stem cell transplants for persons with multiple sclerosis: Is this the best therapeutic option. Medicina Univ 2017; 19: 208-9. DOI: 10.1016/j.rmu.2017.10.003
43. Dobson R, Giovannoni G. Multiple sclerosis – a review. Eur J Neurol 2019; 26 (1): 27-40. doi: 10.1111/ene.13819.
44. Piehl F. Current and emerging disease-modulatory therapies and treatment targets for multiple sclerosis. J Intern Med 2021; 289 (6): 771-791. doi: 10.1111/joim.13215.
45. Kuhlmann T, Moccia M, Coetzee T, Cohen JA, Correale J, Graves J, Marrie RA, Montalban X, Yong VW, Thompson AJ, Reich DS; International Advisory Committee on Clinical Trials in Multiple Sclerosis. Multiple sclerosis progression: time for a new mechanism-driven framework. Lancet Neurol 2023; 22 (1): 78-88. doi: 10.1016/S1474-4422(22)00289-7.
46. Ruiz-Argüelles GJ, Olivares-Gazca JC, Murrieta-Álvarez I, Blumenkron-Marroquin D, González-López E, Ruiz-Arguelles A, Ruiz-Delgado GJ, Gómez-De-León A, Gómez-Almaguer D. Modifications to the “Classical” autologous hematopoietic stem cell transplantation in multiple sclerosis: a less toxic approach is feasible and improves the neurological condition. A Mexican perspective. Biology Blood Marrow Transplant 2018; 24 (3): S125-6. https://doi.org/10.1016/j.bbmt.2017.12.067.
47. Ruiz-Argüelles GJ, Olivares-Gazca JC, Olivares-Gazca M, Leon-Peña AA, Murrieta-Alvarez I, Cantero-Fortiz Y, Gomez-Cruz GB, Ruiz-Argüelles A, Priesca-Marin M, Ruiz-Delgado GJ. Self-reported changes in the expanded disability status scale score in patients with multiple sclerosis after autologous stem cell transplants: real-world data from a single center. Clin Exp Immunol 2019; 198 (3): 351-358. doi: 10.1111/cei.13358.
48. Murrieta-Álvarez I, Cantero-Fortiz Y, León-Peña AA, Olivares-Gazca JC, Priesca-Marín JM, Ruiz-Delgado GJ, Gómez-De-León A, Gonzalez-Lopez EE, Jaime-Pérez JC, Gómez-Almaguer D, Ruiz-Argüelles GJ. The 1,000th transplant for multiple sclerosis and other autoimmune disorders at the HSCT-México program: A myriad of experiences and knowledge. Front Neurol 2021c; 12: 647425. doi: 10.3389/fneur.2021.647425.
49. Olivares-Gazca JC, Sánchez-Bonilla D, Fernández-Gutiérrez JA, Reyes-Cisneros OA, Gallardo-Pérez MM, Ruiz-Delgado GJ, Ruiz-Argüelles GJ. Patient-reported-outcomes and safety of autologous stem cell transplantation in multiple sclerosis: A single center experience with the Mexican method in 1300 persons. Blood 2022; 140 (Supplement 1): 1178-9. https://doi.org/10.1182/blood-2022-163740.
50. Olivares-Gazca JC, Guerrero-Pesqueira F, Murrieta-Alvarez I, Cantero-Fortiz Y, León-Peña AA, Priesca-Marín JM, Gomez-Almaguer D, Gomez-De-Leon A, Ruiz-Delgado GJ, Ruiz-Argüelles GJ. Splitting the total dose of cyclophosphamide in two blocks apart during the conditioning of autologous hematopoietic stem cell transplantation in multiple sclerosis results in diminished cardiotoxicity: Experience in 1,000 patients. Rev Invest Clin 2022b; 74 (1): 1-3. doi: 10.24875/RIC.21000206.
51. Olivares-Gazca JC, Pastelín-Martínez MDL, Montes-Robles MA, Gallardo-Pérez MM, Hernández-Flores EJ, Robles-Nasta M, et al. Can doses of post-transplantation cyclophosphamide in haploidentical stem cell allografts be reduced? Hematology 2023; 28 (1): 2242176. doi: 10.1080/16078454.2023.2242176.
52. Sánchez-Bonilla D, Robles-Nasta M, Gallardo-Pérez MM, Hernández-Flores EJ, Montes-Robles M, Pastelín-Martínez ML, Garcés-Eisele SJ, Olivares-Gazca JC, Ruiz-Delgado GJ, Ruiz-Argüelles GJ. Long-term results of autografting persons with multiple sclerosis are better in those not exposed to prior disease-modifying therapies. Mult Scler Relat Disord 2023; 75: 104744. doi: 10.1016/j.msard.2023.104744.
53. Gómez-Cruz GB, Olivares-Gazca M, Murrieta-Álvarez I, Olivares-Gazca JC, León-Peña A, Cantero-Fortiz Y. À-propos of the 1000th stem cell transplant conducted at the Clínica Ruiz in Puebla. México. Rev Hematol Méx 2019; 20 (2): 150-83.
54. Maziarz RT. Letter to the editor. Rev Hematol Mex 2020; 21 (1): 71.
Received: July 2023
Accepted: September 2023
This article must be quoted: Garces-Eisele J, Olivares-Gazca JC. Pioneering 30 years of hematopoietic stem cell transplants in Puebla, Mexico. Hematol Méx 2023; 24 (2): 37-45.

